Catalytic converter
The catalytic converter consists of a stainless steel housing and is a key component of modern exhaust aftertreatment systems for petrol and diesel engines. It ensures that harmful exhaust emissions from combustion engines are converted into harmless gases.
Function
The catalytic converter is responsible for chemically converting harmful exhaust gases from combustion engines into less harmful substances.
Structure of catalytic converters

A catalytic converter consists of a stainless steel housing that contains either a metallic (metalith) or ceramic (monolith) substrate. This substrate features numerous small longitudinal channels, designed to maximise the surface area for optimal efficiency. The surface of the substrate is coated with a highly porous wash coat layer, into which precious metals, such as platinum, palladium, and/or rhodium are embedded.
Types of catalytic converters
Three Way Catalytic Converter
This type of catalytic converter is designed for petrol engines. When it reaches operating temperature, it converts:
● Unburned hydrocarbons (HC) into carbon dioxide (CO₂) and water vapour (H₂O)
● Carbon monoxide (CO) into carbon dioxide (CO₂)
● Nitrogen oxides (NO, NO₂) into nitrogen (N₂) and oxygen (O₂)
These three processes occur simultaneously, which is why it is called a three way catalytic converter. To function effectively, the catalytic converter requires precise exhaust gas composition. This is achieved when a stoichiometric air/fuel mixture (Lambda = 1) is maintained, meaning 1 part of fuel is mixed with 14.7 parts of air. An oxygen sensor (Lambda sensor), placed between the engine and the catalytic converter, monitors the oxygen content in the exhaust gases. The engine control unit processes this data to regulate the air/fuel mixture.
Modern three way catalytic converters have been improved to reach operating temperature in just a few seconds, a characteristic known as "light-off" behaviour, facilitated by advanced coating technologies. Additionally, some modern designs, such as close coupled catalytic converters, position the converter near the engine exhaust manifold to ensure rapid heat up and efficient emissions reduction.
EOBD-Compatible Catalytic Converters
EOBD (European On-Board Diagnostics) catalytic converters are designed for vehicles that comply with Euro 3 and Euro 4 emissions standards. The EOBD system continuously monitors all emission relevant components and sensors during driving, detecting malfunctions and alerting the driver via a Malfunction Indicator Light (MIL).
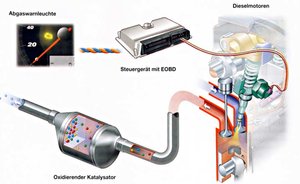
Oxidation Catalytic Converter
Diesel engines operate with an excess of air, resulting in a high oxygen content in the exhaust. The oxidation catalytic converter for diesel engines facilitates two key chemical reactions:
● Carbon monoxide (CO) is oxidised into carbon dioxide (CO₂)
● Hydrocarbons (HC) are converted into carbon dioxide (CO₂) and water vapour (H₂O)
SCR Catalytic Converter
Selective Catalytic Reduction (SCR) catalytic converters are also used in hybrid vehicles, where they reduce emissions from the internal combustion engine. The incorporation of SCR systems in hybrid vehicles helps them meet stringent environmental regulations, making them more attractive to environmentally conscious consumers.
Safety
Catalytic converters using ceramic monoliths require support mats to secure the substrate and prevent damage from vibrations and impacts. Previously, fine ceramic fibres, suspected of being carcinogenic, were used. However, modern "green mats" have been introduced, which are either biodegradable or have fibres too large to be inhaled into the lungs, ensuring improved environmental compatibility.
Depreciation
Catalytic converters are subject to natural ageing. This is partly caused by the high temperatures (sometimes over 800 °C) and mechanical stresses (vibrations) that occur during normal vehicle operation. This can cause the coating of precious metals on the substrate to degrade over time. Today's catalytic converters are assumed to have an average lifespan of 80,000 to 100,000 km.
Apart from bottoming the CAT when driving over raised obstacles, the following factors may also cause premature ageing of the converter:
Catalyst contamination
If the engine burns too much oil, the additives it contains may be deposited on the surface of the catalytic converter, thus sealing it. This stops the exhaust gases coming into contact with the precious metals, meaning the catalytic converter no longer functions properly. Catalyst contamination can also occur if you frequently make short journeys. In this case however, the CAT can often be regenerated by going on a long trip down the motorway. At one time filling up with leaded petrol was a common risk of contamination, but nowadays this can come about only if you happen to be abroad. Take special care when abroad to ensure you fill up only with unleaded fuel (unleaded, sans plomb).
Melting of the catalytic substrate
Faults in the ignition or fuel management system can lead to unburnt air-fuel mixture finding its way to the catalytic converter and burning up on the surface of the CAT. This can cause temperatures in the CAT to exceed 1000 °C, so destroying the substrate. You should therefore go to a garage at once if you notice any faults in the ignition or fuel management system.
Environmental protection
Modern exhaust aftertreatment systems significantly improve air quality by reducing harmful pollutants such as carbon monoxide (CO), hydrocarbons (HC), nitrogen oxides (NOx), sulphur dioxide (SO₂), and particulate matter (PM). Technological advances in emission control ensure that vehicles comply with strict national and international environmental regulations while also providing tax benefits and increasing resale value for vehicle owners.
Katalysator Bilder
- Bild 1: Katalysator ©Eberspächer
- Bild 2: Partikelfilter ©Eberspächer
- Bild 3: Katalysator Schaubild
- Bild 4: Krümmer-Katalysator






